CALCIUM CHLORIDE 94-98% PELLETS MATERIAL SAFETY DATA SHEET
1. IDENTIFICATION OF THE SUBSTANCE/PREPARATION AND OF THE COMPANY/UNDERTAKING
PRODUCT LABEL NAME : Calcium Chloride 94-98% pellets
CHEMICAL NAME : Calcium Dichloride (IUPAC)
SYNONYMS : Calcium (2+) chloride, Calcium (II) chloride, Calcium chloride anhydrous, Calcium dichloride, E509 food additive
USE OF THE SUBSTANCE/
PREPARATION : - Deicer
- Completion fluid and drilling mud additive
- Process chemical - Concrete antifreeze
- Desiccant for drying industrial gases, liquid hydrocarbons and petrochemicals
- Road base stabilizer
- Dust control agent
- Additive in plastics
- pH soil and sewage regulating agent
- Odor control and purification aid
- Tire ballast in agricultural vehicles
- Additive in fire extinguishers
- Freeze conditioning agent for storage and transportation of ores/coal
- Food additive and flavor enhancer E509
- Medication
2. HAZARDS IDENTIFICATION
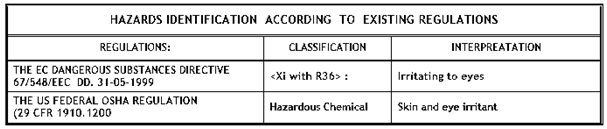
POTENTIAL HEALTH HAZARDS:
INHALATION: Dust may cause irritation of the nose, throat and respiratory tract.
EYES CONTACT : Dust may cause severe eye irritation. May cause corneal injury and conjunctivitis.
SKIN CONTACT: Brief contact is non-irritating to skin. Prolonged contact may cause skin irritation due to abrasion action, even a burn. May cause more severe response if skin is damp or abraded. In the presence of moisture (perspiration, humidity. tears) the dust dissolves to form a solution which may cause burns.
INGESTION: Small amounts swallowed incidentally as a result of normal handling operations are not likely to cause injury. However, swallowing larger amounts causes severe burning and pain in the mouth , throat and abdomen. Vomiting, diarrhea and perforation of the esophagus and stomach may occur.
ENVIRONMENTAL EFFECTS: Calcium chloride is easily dissociated into Ca2+ and Cl- ions in water. Calcium is known as an essential nutrient for higher plants and one of the basicinorganic elements of algae. Calcium strengthens cell walls and plant tissues, reduces toxicity of soluble organic acids, elongating roots. Chloride is also an essential micronutrient for plants and has important roles in he photosynthesis and osmoregulation. The primary cause of the damage to roadside plants is the accumulation of chloride in plant tissues to a toxic level by excess loading of inorganic chloride salts.
3. COMPOSITION / INFORMATION ON INGREDIENTS
4. FIRST AID MEASURES
THE FOLLOWING FIRST AID RECOMMENDATIONS ARE BASED ON ASSUMPTION THAT APPROPRIATE PERSONAL AND INDUSTRIAL HYGIENE PRACTICES ARE FOLLOWED :
IF INHALED : If casualty experiences nausea, headache or dizziness, he should stop work immediately and move to fresh air until symptoms disappear. If breathing is difficult, administer oxygen or artificial respiration, if needed, and GET IMMEDIATE MEDICAL ATTENTION. If respiration or pulse has stopped, have a trained person administer basic life support (artificial respiration, indirect massage of heart, automatic external defibrillator) and CALL FOR EMERGENCY SERVICES IMMEDIATELY.
ON CONTACT WITH EYES : Wash affected eyes with plenty of water for at least 20 minutes. Remove contact lenses, if present, after the first 5 minutes, then continue flushing eyes for at least 15 minutes. If irritation persists, repeat flushing. OBTAIN MEDICAL ATTENTION WITHOUT DELAY, PREFERABLY FROM AN OPHTHALMOLOGIST.
ON SKIN CONTACT : Remove affected clothing and wash contaminated areas thoroughly with soap and water. CALL A PHYSICIAN, if skin irritation persists.
IF INGESTION : If victim is conscious: rinse mouth with water, do not induce vomiting. Give one cup (8 ounces or 240 ml) of water or milk if available and transport to medical facility. Do not give anything by mouth if the person is unconscious. CALL A PHYSICIAN IMMEDIATELY AND TRANSFER THE VICTIM TO HOSPITAL AS SOON AS POSSIBLE.
NOTES TO PHYSICIAN: Due to irritating properties, swallowing may result in burns/ulceration of mouth, stomach and lower gastrointestinal tract with subsequent stricture. Aspiration of vomitus may cause lung injury. Suggest endotracheal / esophageal control if lavage is done. If burn is present, treat as any thermal burn, after decontamination. Medical conditions that may be aggravated by exposure to this product include diseases of the skin, eyes and respiratory tract, preexisting liver and kidney disorders.
5. FIRE-FIGHTING MEASURES
FIRE HAZARD: This product is not flammable and not combustible
SENSIVITY TO STATIC DISCHARGE: Not expected to be sensitive to static discharge.
SUITABLE
EXTINGUISHING MEDIA : Use media appropriate for surrounding fire: Small fires: carbon monoxide, water spray, foam. Large fires: heavy and medium foam or fine water spray.
EXTINGUISHING MEDIA WHICH MUST NOT BE USED FOR SAFETY REASONS : Full water jet.
SPECIAL EXPOSURE HAZARDS ARIZING FROM THE SUBSTANCE: Contact with water may produce heat release. Sealed containers may rapture from the pressure of water vapors released from crystals by intense heat.
HAZARDOUS COMBUSTION PRODUCTS: Thermal decomposition products are toxic and may include hydrochloric acid HCl (CAS 7647-01-0), oxide of chlorine Cl2O (CAS 7791-21-1) and oxide of calcium CaO (CAS 1305-78-8).
PROTECTIVE EQUIPMENT FOR FIRE-FIGHTERS : Fire-fighters should wear positive-pressure self-contained breathing apparatus (SCBA) and protective fire fighting clothing (fire fighting helmet, coat, trousers, boots and gloves). Avoid contact with this material during fire fighting operations. If contact is likely, change to full chemical resistant fire-fighting clothing with self-contained breathing apparatus. It is imperative that firefighters and their equipment are thoroughly decontaminated with a water wash-down after fire and smoke exposure. Machinery and equipment that is involved in a fire must also be decontaminated prior to commencing repair or salvage operation.
OTHER INFORMATION : Remove IBC from fire area if it can be done without risk. Avoid contact with skin. Keep people away. Isolate fire and deny unnecessary entry. This material does not burn. Fight fire for other material hat is burning.
6. ACCIDENTAL RELEASE MEASURES
PERSONAL PRECAUTIONS : For personal protection see Sections 5 and 8. Mark out the contaminated area with signs and prevent access to unauthorized personnel. Use appropriate safety equipment: respirator, protective clothing and gloves. Spilled material may cause floors and contact surfaces to become slippery. Minimize air borne spreading of dust. Refer to Section 7 for additional precautionary measures.
ENVIRONMENTAL PRECAUTIONS : Knock down dust with water spray jet. Prevent from entering into soil, ditches. Keep out of drains and water courses. See Section 12 <Ecological Information>.
METHODS FOR CLEANING UP : Small and large spillages: Collect spilled material in suitable and properly labeled containers. Avoid dry sweeping. Do not use compressed air to clean surfaces. Vacuuming or wet sweeping is preferred. Flush residue with plenty of water. Refer to Section 13 <Disposal Considerations> for additional information.
7. HANDLING AND STORAGE
HANDLING : For industrial or professional use only. Usual safety precautions for handling chemicals should be observed. Avoid breathing dust, avoid contact with eyes, skin and clothing. Do not swallow. Wash hands thoroughly after handling. Heat developed during dilution or dissolving is very high. Use cool water (<27oC / 80oF) when diluting or dissolving. Keep container tightly closed.
TECHNICAL MEASURES : Use only with adequate ventilation. Use the product in closed system (transfer by pump or gravity), handle small quantities under a lab hood. Ventilation should be corrosion proof.
FIRE PREVENTION MEASURES : Keep away from incompatible products.
STORAGE :
CONDITIONS OF STORAGE : Store the product in its original tightly closed container in a dry, cool, well-ventilated place, away from heat, spark and open flame. Protect from atmospheric moisture. Prolonged storage may cause product to cake and become wet.
ADVICE ON STORAGE COMPATIBILITY: Do not store together with animal feedstuffs. Do not store together with food.
SPECIAL ELECTRICAL EQUIPMENT: Provide tight electrical equipment well protected against corrosion.
INCOMPATIBLE MATERIALS: Equipment for storage, handling or transportation should not be made of brass, zinc, mild steel, aluminum and its alloys, iron and its alloys.
PREVENTION OF STATIC ELECTRICITY : Ground all equipment
TYPE OF MATERIAL USED IN THE PACKING / CONTAINERS: Materials of construction for storing the product include: 304 stainless steel, titanium and polyethylene.
SPECIFIC USE(S): For further information, please check up supplier’s Technical Data Sheet
8. EXPOSURE CONTROLS / PERSONAL PROTECTION

MAC = Maximum Allowable Concentration
TLV = Threshold Limit Value is available from the American Conference of Governmental Industrial Hygienists (ACGIH)
REL = Recommended Exposure Limit published by The National Institute for Occupational Safety and Health (NIOSH).
PEL = Permissible Exposure Limit, legal limit for occupational exposure to individual chemical adopted by OSHA
STEL = Short Term Exposure Limit
TLV/TWA= Threshold Limit Value / Time Weighted Average
EXPOSURE CONTROLS :
TECHNICALS MEASURES : Minimize creation of dust. To control airborne levels below the exposure guidelines, provide either general, or local exhaust ventilation, closed design equipment and regular cleaning of production rooms. Ventilation should be corrosion proof. If air is to be recirculated, it must be filtered properly. Ventilation should be corrosion proof. Ventilate low lying areas such as sumps and pits where dense dust may collect.
MONITORING PROCEDURES : Regular exposure limits monitoring
RESPIRATORY PROTECTION : Avoid breathing dust or aerosol. Use only with adequate ventilation. A respirator is not required if local/general exhaust ventilation is adequate. In the case of aerosol formation, use respirator with an approved filter [type P2 for European member states] or respirator with N95 (fume, mist) cartridges [USA, Canada]. Apply self-contained breathing apparatus when the mask and cartridge do not give adequate protection.
HAND PROTECTION : Wear impervious chemicals resistant gloves made of Neoprene, PVC, Nitrile/Butyl rubber (>480 min, EN374).
EYE PROTECTION : Use good industrial practice to avoid eye contact. For dusty operations or when handling solutions of the material, wear appropriate chemical safety goggles with full face shield as described by OSHA’s eye and face protection regulations in 29 CFR 1910.133 or European Standard EN166. Ensure eye bath is at hand. Contact lenses should not be worn when working with this material.
SKIN PROTECTION : Wear clean, body-covering clothing. Launder contaminated clothing and clean protective equipment before reuse. Have safety shower available at locations where skin contact can occur.
HYGIENE MEASURES : General industrial hygiene regulations are to be observed. Do not consume or store food in the working area. Wash hands before smoking or eating.
9. PHYSICAL AND CHEMICAL PROPERTIES
9.1. GENERAL INFORMATION
Appearance Pellets
Color White
Odour Odorless
9.2. IMPORTATNT HEALTH, SAFETY AND ENVIRONMENTAL INFORMATION
pH value : 4,5 - 8,5 @ 5% aqueous solution
8,0 - 9,0 @ 35% aqueous solution
Boiling point : > 1600oC / 2912oF
Flash point Not applicable.
Flammability : The product is not flammable.
Explosive properties : Not applicable
Oxidizing properties : Non oxidizer
Vapour pressure: 0,005 mm Hg @ 20oC / 68oF
Density 2,16 g/cm3 / 134,84 lb/ft3 @ 25oC / 77oF
Water solubility : 745 g/l @ 20oC / 68oF
59,5% by weight @ 0oC / 32oF
Soluble in ethyl alcohol, acetone and acetic acid
Partition coefficient: n-octanol / water : Not applicable
Viscosity Not applicable
Vapour density (Air = 1.0): Not applicable
Evaporation rate (Butyl Acetate = 1.0): Not applicable
9.3. OTHER INFORMATION
Bulk density, min 0,81 - 0,88 g/cm3 / 50,57 - 54,94 lb/ft3
Melting point 772oC / 1422oF
NOTE: These physical data are typical values based on material tested but may vary from sample to sample. Typical values should not be construed as a guaranteed analysis of any specific lot or as specifications for the product.
10. STABILITY AND REACTIVITY
STABILITY : Hygroscopic. Stable under recommended storage and handling conditions indicated in Section No.7.
HAZARDOUS POLYMERIZATION : Does not occur.
CONDITIONS TO AVOID : Avoid moisture, high temperature, sparks and open flames. Minimize air borne spreading of dust. Sweep up immediately to eliminate slipping hazard.
MATERIALS TO AVOID : Water: Heat is generated when mixed with water. Spattering and boiling can occur. Acids: Yields hydrogen chloride (CAS 7647-01-0) in contact with sulphuric acid and other mineral (boric acid, etc.) acids, including Lewis acids. Metals: Flammable hydrogen (CAS 133-74-0) gas may be generated from contact with metals such as zinc, aluminum, tin and lead.
Reacts violently with such metals as sodium, potassium and barium, particularly if they are finely divided. Bromine trifluoride: Reacts violently Explosive when mixed with Furan 2-peroxycaboxylic acid
HAZARDOUS DECOMPOSITION PRODUCTS : Will react with some metals (zinc, aluminum, tin, lead, alkali metals) forming flammable hydrogen (CAS 133-74-0) gas.
HAZARDOUS COMBUSTION PRODUCTS: Thermal decomposition products are toxic and may include hydrochloric acid (CAS 7647-01-0), oxide of chlorine Cl2O (CAS 7791-21-1) and oxide of calcium CaO (CAS 1305-78-8).
11. TOXICOLOGICAL INFORMATION
HEALTH EFFECTS:
MAIN EFFECTS: The product causes buns of eyes, skin and mucous membranes. The seriousness of the lesions and the prognosis of intoxication depend directly on the concentration and duration of exposure.
ROUTES OF EXPOSURE: Refer also to Section 4 of this MSDS fro routes of exposure and corresponding symptoms
EYES CONTACT : Calcium chloride, as an aerosol, mist or vapor, irritates eyes. Contact causes severe irritation, corneal damage and impairment of vision.
INHALATION : Inhalation will cause severe irritation of nose, throat and lungs. It may also cause burns to the respiratory tract, which can result in shortness of breath, wheezing, choking and chest pain. SKIN CONTACT : The extent of injury depends on the duration of contact. Dermal exposure may cause severe irritation and/or burns characterized by redness and swelling.
INGESTION : Ingestion may cause a burning sensation in the mouth, irritation, corrosion / ulceration of the entire gastrointestinal tract, diarrhea, bleeding, abdominal pain and vomiting. Asphyxia can occur.
CHRONIC EFFECTS FROM LONG-TERM EXPOSURE :
INHALATION: Chronic (repeated) inhalation exposure may cause cough, running nose, bronchopneumonia, pulmonary edema (fluid build-up) and reduction of pulmonary function. During long-term exposure calcium accumulates in the organism, milk-alkali syndrome develops and conjunctivitis occurs.
EYES CONTACT: After several days of exposure, ulceration and corneal opacification may come, which leads to blindness.
SKIN CONTACT: Permanent skin exposure causes scab formation. Prolonged, confined (especially under the finger nails, under rings or watch bands) or repeated exposure may cause skin irritation and possibly lead to chemical burns.
INGESTION: There may be corrosion of the lips, mouth, tongue and pharynx, bleeding from the mouth or nose. Perforation of the alimentary tract and constrictive scarring may result. Oesophageal stricture may occur week, months or even years later to possibly make swallowing difficult. The estimated fatal dose for men is 5 grams.
MEDICAL CONDITIONS AGGRAVATED BY EXPOSURE: Asthma, respiratory and cardiovascular disease
SENSITIZATION : Did not cause sensitization.
CARCINOGENICITY : The ingredients of this product are not classified as carcinogenic by EPA,
ACCORDING TO ANNEX II OF EC REGULATION 1907/2006 DD. 18.12.2006
IARC, NTP, OSHA or ACGIH.
MUTAGENICITY : Is not known or reported to be mutagenic.
TERATOGENICITY DATA: No adverse teratogenic effects are anticipated.
TOXICITY FOR REPRODUCTION : Not applicable.
12. ECOLOGICAL INFORMATION
ACUTE ANIMAL TOXICITY DATA:
ORAL TOXICITY: is low for animals, due to the severe irritating property of the original substance or its high-concentration solutions to the gastrointestinal tract: LD50 (Rat): 3798 - 4179 mg/kg LD50 (Mice): 1940 - 2045 mg/kg LD50 (Rabbit): 500 - 1000 mg/kg is rare in humans, because large single doses induce nausea and vomiting
DERMAL TOXICITY: LD50 (Rabbit): > 5000 mg/kg
SKIN IRRITATION: Rabbit: Slightly irritating
EYE IRRITATION: Rabbit: Irritating
ACUATIC TOXICITY: Product is practically non-toxic to aquatic organisms on an acute basis: LC50 > 100 mg/l in the most sensitive species tested.
FISH ACUTE TOXICITY: LC50 (Bluegill) : 8350 - 10650 mg/l
LC50 (Pimephales promelas) : 4630 mg/l @ 96 hrs
LC50 (Gambusia affinis): 13400 mg/l @ 96 hrs
CRUSTACEANS ACUTE TOXICITY: EC50 (Daphnia magna): 1062 mg/l @ 48 hrs (immobilization)
EC50 (Tubiflex tubiflex): 780 mg/l @ 96 hrs (immobilization)
ALGAE ACUTE TOXICITY: EC50 (Selenastrum capricornutum): 2900 mg/l @ 72 hrs (biomass)
TOXICITY TO MICRO-ORGANISMS: EC50 (Activated sludge, respiration inhibition) > 1,000 mg/l CHRONIC ACUATIC TOXICITY: more than 100 mg/L: EC16 (Daphnia magna): 330 mg/l
MOBILITY : The high water solubility (745 g/l @ 20oC / 68oF) indicates that in environment Calcium Chloride will be found predominantly in water. In water (including soil or sediment pore water), the product exists as the Calcium ion (Ca2+) and chloride ion (Cl-) dissociated in water.
AIR : Chemical degradation
WATER : Considerable solubility and mobility.
SOIL / SEDIMENTS: The Calcium ion in soil may bind to soil particulate or may form stable inorganic salts with sulphate and carbonate ions. The Chloride ion is mobile in soil and eventually drains into surface water because it is readily dissolved in water.
PERSISTENCE AND DEGRABILITY :
ABIOTIC DEGRADATION:
AIR : No data
ATMOSPHERIC HALF-LIFE: No data
WATER: Neutralization
SOIL: Ionization / neutralization
BIODEGRADATION : The methods for determining the biological degradability are not applicable to inorganic substances.
BIOACCUMULATIVE POTENTIAL : Bioaccumulation in organisms is not relevant for Calcium chloride.
RESULTS OF PBT ASSESMENT: This product is not considered as PBT or vPvB substance.
PERSISTENCE: Calcium Chloride rapidly dissociates in water. Therefore it does not fulfill the
ACCORDING TO ANNEX II OF EC REGULATION 1907/2006 DD. 18.12.2006
P criterion.
BIOACCUMULATION: Bioaccumulation in organisms is not relevant for Calcium Chloride solution, therefore, this product does not meet the B criterion of the PBT criteria.
TOXICITY: Calcium Chloride does not meet the T criterion in the PBT assessment.
13. DISPOSAL CONSIDERATIONS
SAFE HANDLING OF RESIDUES:: Product waste must follow applicable federal, state and local regulations. For unused or uncontaminated product, the preferred options include either sending to a licensed, permitted recycler / incineration or other thermal destruction device or industrial landfill or dilution with plenty of water or neutralization with acid before discharge.
PACKING DISPOSAL : Empty containers and clean them with water. Dispose of an unused product. The empty and clean containers are to be reused in conformity with regulations.
Local, state, provincial, and national disposal regulations may be more or less stringent. Consult your attorney or appropriate regulatory officials for information on such disposal.
European Waste Catalogue (EWC)
Decision (2000/532/EC) / EWC): Waste product Code No. 06 09 04 <Calcium-based reaction wastes other than those mentioned in 06 09 03>
14. TRANSPORT INFORMATION
LAND TRANSPORT:
ADR/RID CLASS: Not classified as Dangerous Goods
DOT(USA) / TDG(CANADA) CLASS: Not regulated
UN NUMBER: Not available
PROPER SHIPPING NAME: Calcium Chloride 94-98%, pellets
SEA TRANSPORT :
IMO/IMDG CODE : Not classified as Dangerous Goods MARINE POLLUTANT : No UN NUMBER: Not available PROPER SHIPPING NAME : Calcium Chloride 94-98%, pellets
AIR TRANSPORT:
ICAO/IATA CLASS: Not classified as Dangerous Goods
UN NUMBER: Not available
PROPER SHIPPING NAME : Calcium Chloride 94-98%, pellets
15. REGULATORY INFORMATION

OTHER REGULATIONS :
CANADA REGULATIONS:
WHMIS CLASSIFICATION: D-2B: Toxic Material Causing Other Toxic Effects (Skin and Eye Irritant).
USA REGULATIONS:
US EPCRA SARA TITLE III Extremely Hazardous Substance (40CFR 355, Appendix A):
SECTION 302 not regulated
US SARA HAZARD DESIGNATION (SARA 311/312): Acute Health Hazard: Yes.
US SARA TITLE III RULE: (SECTION 313 SUPPLIER NOTIFICATION): This product does not contain chemicals at levels which require reporting under this statute.
US RCRA (RESOURCE CONSERVATION AND RECOVERY ACT) STATUS : Not a RCRA waste.
US CERCLA SECTIONS 102a/103 HAZARDOUS SUBSTANCES (40CFR 302.4): None of the chemicals in this material have a reportable quantity (RQ).
OSHA HAZARD
COMMUNICATION STANDARD: Mild eye irritant.
EUROPEAN REGULATIONS: Export and Import of Dangerous Chemicals EC Regulation No.689/2008: Not listed in Annex I of EC Regulation No.689/2008
EUROPEAN WASTE CATALOGUE (EWC) DECISION (2000/532/EC): Waste product Code No. 06 09 04 <Calcium-based reaction wastes other than those mentioned in 06 09 03>
GERMAN WGK class: Water hazard class 1: slightly hazardous for water.
THE RUSSIAN FEDERATION REGULATIONS: Russian Federation Law «On Consumer's Right Protection», Pollution Control Regulations, Sanitary Epidemic Control, «On Technical Regulation»
16. OTHER INFORMATION
RECOMMENDED
RESTRICTIONS ON USE: For industrial or professional use only.
MAIN APPLICATIONS : 1. De-icer for sidewalks, parking lots and road treatment (CaCl2 absorbs water and forms solutions with very low freezing points, ice melting is also accelerated by exothermic (heat releasing) nature of water absorption process), calcium chloride is also used to freeze-proof sand that is spread on icy roads
2. Industrial Processing: additive in plastics, calcium salts production, component of organic fertilizer preparations (neutralizes soil pH, reduces soil crusting, aids plant growth, assists in water retention, protects plants from diseases such as sclerotinia), sewage pH,odor control and purification aid [removes phosphates and fluorides (main sources: petroleum refineries, aluminum smelters, semi-conductor production facilities) from wastewater]
3. Stabilization of highway and road construction (binds chemically clay particles, reduces soft spots, inhibits freezing and frost heaves), environmentally friendly dust suppressants on unpaved roads, parking lots, sport grounds and in mining industry
4. Oil and Gas drilling: completion and work-over fluids, oil based mud fluids (boosts the efficiency of drilling due to increased density of fluid), pipeline inspection cleaning and hydro testing
5. Concrete additive: acts as concrete antifreeze during fall and winter projects and shortens the time of setting due to increased rate of hydration
OTHER APPLICATIONS:
1. Liquid tire ballast in agricultural vehicles: increases tractor traction, improves drawbar pull, extends the life of tires by hydra-inflating their surface
2. Mining: freeze conditioning agent for winter storage and transportation of ores/coal (1-2l per ton)
3. Antifreeze for curling and skating rinks
4. Cost-effective desiccant for drying industrial gases and liquid hydrocarbons at refineries (diesel, jet fuel, propane, butane, ethane, LPG) and petrochemicals (mixed C4s, chlorinated/aromatic hydrocarbon, acetylene)
5. Food industry: food additive and flavor enhancer E509 (provides salty taste to pickles and maintains firmness of canned vegetables) with approved average intake 160-345 mg/day for individuals, post harvest dip to increase shelf life of fruits and vegetables, corrects mineral deficiencies and enhances flavor of soft drinks and beer, refrigerant in ice cream and frozen desert manufacturing, serves as a meat tenderizer, dip in calcium chloride inhibits acrylamide carcinogen formation in potato chips and fries, restores the natural balance between Ca and proteins in milk for the purpose of making brie or stilton cheese, substitutes salt in animal feed as a supplement or for calcium deficiency, for example in dairy cow feeding)

